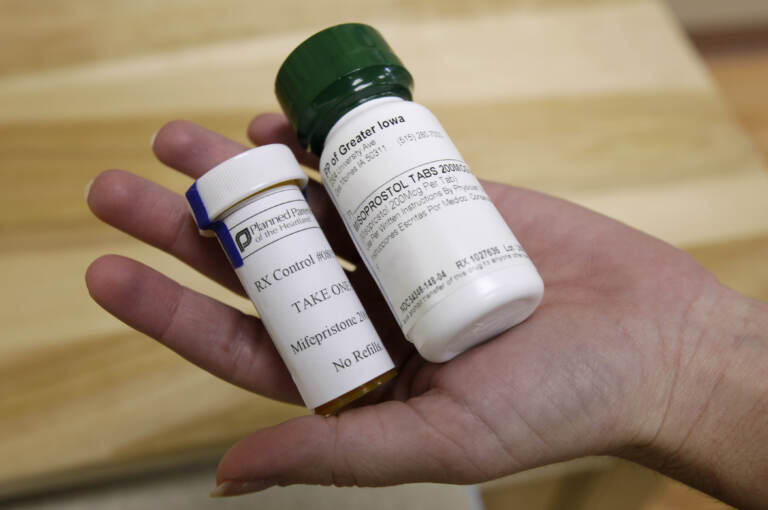
Washington: The US Food and Drug Administration (FDA) approved a program that allows brick-and-mortar retail pharmacies to offer abortion pills directly to patients, a manufacturer of the drug said in a press release.
“Danco Laboratories is pleased to announce that on January 3, 2023, the FDA approved Risk Evaluation and Mitigation Strategy (REMS) modifications to the Mifepristone REMS Program,” the release said on Tuesday. “Pharmacies who become certified in the Mifepristone REMS Program may dispense Mifeprex directly to patients upon receipt of a prescription from a certified Mifeprex prescriber.”
The pro-abortion Planned Parenthood organization in a tweet said it applauds the FDA’s “important step forward.” The group also said eliminating “medically unnecessary” restrictions is critical for improving access to abortions.
Dispensing of the drug was previously restricted to a few mail-order pharmacies or specially certified doctors or clinics, The New York Times reported in an article re-tweeted by the White House.
Demand for the pill in the US has increased, the report added, since the Supreme Court overturned Roe v. Wade last June, which ended the federal constitutional right to an abortion.
Most types of abortions are now banned in thirteen states.